What is Emergency Use Authorization?
EUA or Emergency Use Authorization is an authority given to the Food and Drug Administration (FDA) to approve the use of drugs or medical devices for an emergency indication in the US.
The Emergency Use Authorization (EUA) authority permits the FDA to assist the public health protections of the country against chemical, biological, radiological & nuclear (CBRN) threats. During a medical emergency, the EUA protects the public health by facilitating the availability as well as the use of medical countermeasures (MCMs) that are required during public health emergencies.
The EUA is given to drugs as well as medical devices for any emergency medical indication.
Why is a EUA issued?
EUA is an essential tool for physicians as well as for public health officials who are involved in an emergency response. The Emergency Use Authorization can enable them to use the best countermeasure available for detection, prevention, or treatment of a disease or indication in specific populations. With the EUA, the countermeasure can be used in a medical emergency if it is not approved by the FDA or not approved for that particular use.
The FDA issues Emergency Use Authorizations. Therefore, these authorizations reflect the mission of FDA to protect public health by ensuring the safety, efficacy, as well as security of human & veterinary drugs, medical devices, biological products, the supply of food, cosmetics, and products that emit radiation in the US.
Criteria for Issuance of EUA
After the required determination and declaration have been issued, following feasible & appropriate consultations, the FDA may issue a EUA. The FDA issue the EUA only when the agency concludes that the following four statutory standards for issuance have been met-
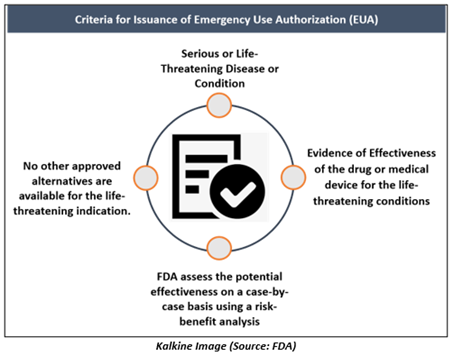
- Serious or life-threatening indication- For FDA to issue a EUA, the CBRN agents referred to in the EUA declaration of HHS Secretary must be capable of triggering a life-threatening or severe condition.
- Evidence of Effectiveness- For a EUA, it is the specific requirement that the proposed medical countermeasures may be efficient to prevent, diagnose, or treat serious or life-threatening diseases. The FDA plans to assess the potential effects on a case-by-case basis using a risk-benefit analysis.
- Risk-Benefit Analysis- For determination of whether the known & potential benefits of the product outweigh the risks, the FDA will consider the totality of the scientific proof. Evidence could arise from a variety of sources available for FDA consideration, such as data from clinical trials, animal studies including in vitro data.
- No Previously-Approved Alternatives- A EUA can be issued if there are no existing approved alternatives for the life-threatening indication.
What is the process of EUA?
For Emergency Use Authorization FDA evaluates the options very quickly using the available evidence. Also, before giving EUA, the FDA carefully checks for any known, potential risks as well as benefits of making the drug or medical device available during the emergency.
The EUA process comprises the following steps-
- Determination and declaration of a health emergency.
- Submission of EUA request.
- Review of the EUA request by the FDA.
- A EUA issuance or rejection of the request.
- Termination of EUA after the medical emergency ends.
Generally, the FDA review and action timelines on a request to grant a EUA shall be determined based on specific cases. The timeline will depend on several factors including-
- The product profiles.
- The existence of pending applications for the product, if any.
- The type of the emergency, potential emergency, or threat of emergency.
- The organization & totality of the request submission.
- The workload of the personnel of reviewing Center.
The development pathway for pharmaceuticals undergoes pre-investigational new drug (pre-IND), IND, and new drug application (NDA). The NDA comprises how a drug manufacturer formally propose that the FDA authorize the sale and marketing of a new drug.
However, for EUA, it is not required that products be in a specific point of the development pathway. But for emergency authorization, it is indicated that the product is under development or has been developed.
What are the conditions for EUA?
The Food and Drug Administration may establish restrictions on a EUA, necessary or appropriate for the protection of public health. Below are the few examples of what can be addressed within the conditions of authorization-
- Information relating to the EUA Product.
- Information for Health Care Professionals (HCPs) or authorized dispensers.
- Recipients information.
- Monitoring and reporting of ADRs (adverse drug reactions).
- Records- As far as possible given the conditions of the emergency, FDA must establish conditions for a manufacturer of to keep records and to grant FDA access to records concerning the EUA product.
- Restrictions on distributing and administration- There may be some conditions on how the administration and distribution are to be done.
- Advertising limitations.
Emergency Use Authorization for the ongoing life-threatening disease, COVID-19
The Department of Health & Human Services has announced a public health emergency that supports the emergency use of remdesivir for treatment of COVID-19. In response, the US FDA has issued a EUA for remdesivir for COVID-19 treatment.
US-based biopharmaceutical company Gilead Sciences Inc (NASDAQ:GILD) is developing remdesivir for treatments of COVID-19. On 1 May 2020, remdesivir received Emergency Use Authorization by the US FDA for treatment of severe COVID-19; and on 28 August 2020, the FDA expanded the Emergency Use Authorization facilitating the use of Veklury® (remdesivir) to treat moderate COVID-19 patients.
To know more about how remdesivir works against COVID-19, Click here.
 Please wait processing your request...
Please wait processing your request...