What are clinical trials?
Clinical trials are experiments on human volunteers to evaluate the safety and efficacy of a new medical treatment. In other words, clinical trials are carefully designed studies which test the benefits along with risks of a specific medical therapy or intervention, such as a new chemical entity.
The trials are conducted by healthcare institutions and pharmaceutical/biotech companies, usually in collaboration. The studies are headed by a lead investigator, known as principal investigator. Early-stage studies are conducted at a couple of sites in a single geography while late-stage trials are carried out at multiple locations across different geographies.
Several healthcare and biotech companies perform clinical trials in collaboration with institutes or universities. Large-cap healthcare players, with a market capitalisation greater than US$10 billion, generally have the geographical presence and financial capability to conduct large-scale clinical trials on their own. Small-cap companies, however, have limited resources and typically enter license agreements with their bigger counterparts to conduct large-scale trials.
What are the various phases of clinical trials?
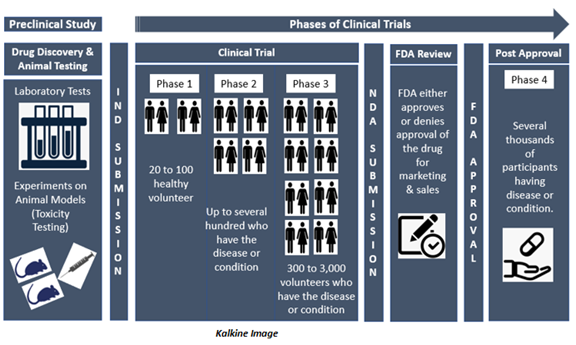
To understand the various phases of clinical trials, let us the example of the biggest healthcare market in the world, the US. After scientists and researchers complete the rigorous screening and preclinical testing process, the Company files for an Investigational New Drug (IND) application with the US Food and Drug Administration (FDA). After approval of this application by the FDA, the investigational drug can be tested in human volunteers under clinical trials.
If the preclinical research findings are promising, the FDA approves the IND application of the drug for clinical trials in human volunteers. Clinical trials take place in several phases that are discussed below-
Phase 0- Phase 0 clinical trial is conducted with a minimal number of people, usually fewer than 15. In this phase, the clinical trial investigators use a very small dose of the drug to make sure it is not harmful to human beings before they commence next stage studies with higher doses of the drug.
If there is a case that the drug or treatment acts differently than anticipated, the investigators will likely do additional preclinical research before deciding whether to continue the trial.
Phase 1- During Phase 1 clinical trial, investigators spend several months looking at the effects of the medication on about 20 to 100 healthy volunteers.
This phase mainly aims at figuring out the maximum dose humans can take without any serious side effects. Investigators examine volunteers carefully to observe how they react to the drug during this phase. In addition to evaluating the safety and ideal dosage, investigators also look at the best way to administer the drug, like orally, intravenously (IV), Intradermally or topically, among other routes.
According to the FDA, nearly 70% of medications move on to Phase 2 clinical trial.
Phase 2- This phase of the clinical trial involves several hundred volunteers who have the disease or condition that the new drug is intended to treat. In Phase 2 clinical trial, usually the same dose, that was found to be safe in Phase 1, is administered to the participants.
Investigators examine participants for several months or years to find out how effective the treatment is and collect more information about any side effects the new drug might cause.
While Phase 2 involves more participants than in earlier phases, it is still not large enough to demonstrate the overall safety of a medication. However, the information collected during this Phase help researchers come up with methods and procedures to conduct Phase 3 trials.
As per the FDA, approximately 33% of medications move on to Phase 3 clinical trial.
Phase 3- Phase 3 clinical trials usually involves 300 to 3,000 volunteers having the condition or disease that the new drug/treatment is intended to treat. Some studies can even have participants in five digits depending on the prevalence of the disease being studied. Phase 3 clinical trials can last for several years (from 1 to 4 years).
The main objective of Phase 3 clinical trials is to assess how the new drug works in comparison to already existing medications for the same disease. To go ahead with the clinical trial, investigators are required to demonstrate that the medication is at least as safe & effective as existing treatment options.
The FDA estimates that approximately 25-30% of drugs move to the Phase 3 clinical trial.
Phase 4- Phase 4 clinical trials take place after the drug is approved by the FDA. The trial involves monitoring the safety and efficacy of a device or a drug in a large population. In Phase 4 clinical trial, investigators get more information about the long-term safety, effectiveness, and any other benefits of the approved drug.
The selection of participants, age groups, sex, ethnicity etc. for the clinical trials is based on the disease or condition being studied. As an example, for diseases where prevalence is high among the old age population, a higher number of baby boomers will be enrolled in the studies.
What are the benefits and risks involved for the volunteers?
All clinical trials have risks as well as benefits. The risks of participating in clinical trials depend both on the treatment being evaluated and the health of the participants. Clinical researchers will describe the well-known risks before commencing the trial when a participant receives an informed consent form.
The volunteers will be informed by the researchers if they find any new risk information during the trial.
The risk involved during the trials are the same as routine medical care, and the activities of daily living do. When assessing the risks of research, one can consider two factors:
- Chance of any harm occurring during clinical trials.
- The level of harm that could result from participating in the clinical trials.
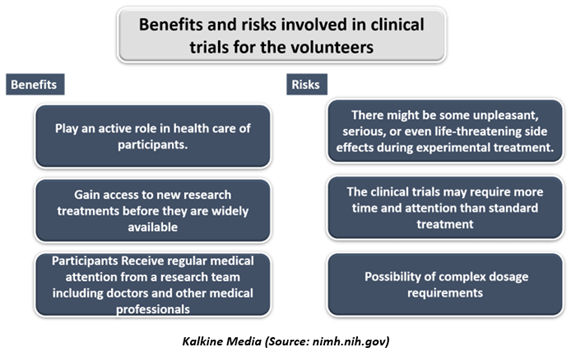
Every clinical trial has its benefits and risks, depending on the type of trial and what it is trying to achieve.
Potential Benefits- Well-designed and properly executed clinical trials offer the best approach for participants to-
- Get access to a treatment that isn't available yet- This treatment may be more effective or have fewer side effects than the treatments that are currently available.
- Volunteers could perform an active role in their health care.
- The clinical trials gain access to new research for treatments before they are widely available in the market.
- During the clinical trials, participants receive consistent and careful medical attention from a research team of doctors and other health professionals. The treatment may be free or at a lower cost.
- Contributing to the clinical trial might save lives in the upcoming time by contributing to medical research.
Potential Risks- Risks for volunteers during the clinical trials include:
- During the clinical trials, there may be unpleasant, severe, or even life-threatening side effects to investigational drug/treatment.
- The clinical trials may require more time and attention than standard treatment would, such as visits to the study site, several blood tests, more treatments, hospital stays, or complex dosage requirements.
- The new treatment may not work for everyone, even if it is beneficial for some other volunteer in the trial. It may be possible that the new medication is not as effective as the currently available treatment.
- Volunteers are not being able to choose which drug/treatment they are going to receive during the trial. In randomised trials, volunteers are randomly assigned to get any particular treatment.
In some trials, subjects may be assigned to get a placebo or sugar pills. In a randomised, double-blinded study, neither the volunteer nor the investigator would know on which treatment the testing is being performed (although the information is available if it is needed).
What are the steps after each phase of clinical trials?
Once a clinical trial or study has completed, the investigators or researchers will carefully collect and analyse the data to find out what next steps are required as an outcome of the findings.
Before a trial starts, the participants should be provided information about how long it is going to last, whether participants will continue receiving the study treatment after the trial ends, if applicable, and how people will be kept informed about the results of the study.
 Please wait processing your request...
Please wait processing your request...