What is Accelerated Approval?
Accelerated approval is an approval pathway regulated by the Food and Drug Administration (FDA) that allows an early approval of treatment or drugs that are used in the treatment of severe or life-threatening indications and fulfill an unmet medical need.
Accelerated approvals by the FDA depend on the assessment of surrogate endpoints as a clinical benefit measure to the patients. Therapies or treatments meeting the standards for traditional approval are not applicable for getting an accelerated approval.
FDA grants accelerated approval in situations when an indication course is prolonged and clinical benefit measurement would require substantial time. For example, therapies used for treating specific tumors or HIV, where an impact on the growth of a tumor or viral load can be identified promptly, but the effect on survival is long-term because of the typical course of the disease.
There is one more scenario in which accelerated approval is applied, that is for acute disorders. For which an extensive study would be required to demonstrate clinical benefit because the occurrence is rare, although a surrogate endpoint can be utilized in much lesser duration. Hence, this leads to a faster study.
Accelerated approval is a part of the drug development process, making a drug available as soon as possible. The other three approaches are- priority review, breakthrough therapy designation and fast track designation.
Which treatments qualify for accelerate approval?
There are some risks associated with the accelerated approval process. These risks explain why the approval is restricted to the treatments showing an effect on an endpoint that is expected to predict clinical advantage and that meet the below-mentioned requirements-
- A drug that is for treatment of a severe or life-threatening indication.
- Offer a meaningful therapeutic value over already existing treatments.
- Shows an effect on an endpoint that is likely to predict the clinical advantage of the drug.
For drugs that have granted accelerated approval, post-marketing confirmatory trials have been necessary for verifying and describing the expected effect on irreversible morbidity or mortality (IMM) or other clinical advantages.

Potentially, accelerated approval is also useful in acute indications where the intended clinical benefit can be shown only in a large scale study because the clinical outcome that would need to be assessed for demonstrating clinical benefit occurs rarely.
For instance, accelerated approval could be for the treatment of an acute disorder where an effect related to the surrogate endpoint can be demonstrated in a few patients. However, a large scale analysis would be required to indicate the impact on clinical findings, like survival.
Conditions for accelerated approval
Therapies or treatments granted accelerated approval should meet similar effectiveness as well as safety standards as treatments undertaking traditional approval.
Moreover, this decision is a judgement call based on the biological plausibility as well as clinical data indicating that relationship.

Additionally, the company that is applying for an accelerated approval should meet the subsequent requirements-
- Copies for all the promotional materials to be used need to be submitted.
- Perform post-marketing, confirmatory clinical studies for the verification of the expected clinical effect.
A company preparing for obtaining accelerated approval should begin conversations with the review department of the FDA during the process of development and offer information that supports the use of the selected surrogate endpoint along with the intended confirmatory experiments.
What are the endpoints for Accelerated Approval?
According to the FDA, the two categories of endpoints are applicable as a basis for accelerated approval are-
- The surrogate endpoint considered to predict any clinical benefit.
- The clinical endpoint which could be assessed earlier than IMM that is likely to foresee any effect on IMM or additional clinical benefit.
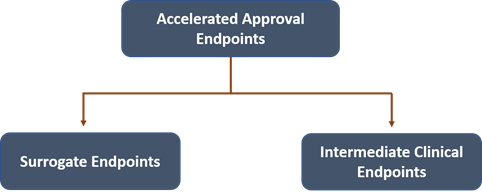
A clinical endpoint precisely measures the therapeutic effect of a treatment, an effect on in what way a patient feels, functions, or survives.
What are the Benchmarks for a drug to get Accelerated Approval?
According to the FDA, drugs or treatments granted accelerated approval should fulfill similar statutory standards for safety as well as effectiveness as for granted traditional approval.
For effectiveness, the standard is significant proof based on satisfactory as well as properly-controlled clinical examinations.
For safety, the standard is getting adequate information for the determination of the safety of the drug for use under prescribed conditions, suggested or recommended in the proposed labeling. In accelerated approval, the Food and Drug Administration can depend on particular evidence, like the effect of a treatment or drug on a surrogate endpoint, as a basis for granting the approval.
Moreover, the application for accelerated approval should also comprise supporting documents for an intermediate clinical endpoint or a proposed surrogate endpoint that it is relatively likely to foresee the intended clinical advantage of treatment.
Risks associated with accelerated approval
There are some risks associated with the accelerated approval; the main risks include the following-
- Surrogate endpoints are used for the reason that they are assumed to predict clinical advantage.
- Fewer, shorter, as well as smaller clinical trials, resulting in less information about rare or delayed adverse events.
When can the withdrawal of accelerated approval take place?
The FDA has the authority to withdraw accelerated approval for the subsequent reasons-
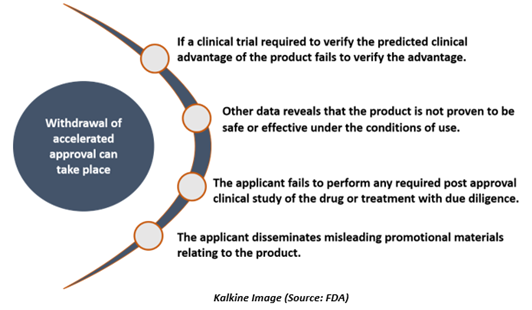
The FDA could withdraw drug approval if the clinical trials do not validate the clinical benefit or do not demonstrate any sufficient clinical benefit for justification of any risk associated with the drug.
There is no specified timeline for FDA response for Accelerated Approval.
 Please wait processing your request...
Please wait processing your request...