AAPL 189.72 1.2218% MSFT 423.08 1.5652% GOOG 173.88 1.1342% GOOGL 172.51 1.2739% AMZN 185.99 -0.5773% NVDA 946.3 3.5838% META 481.54 2.0536% TSLA 173.99 -2.0051% TSM 155.58 2.3889% LLY 787.02 3.1914% V 281.5 1.3538% AVGO 1436.17 4.068% JPM 202.11 0.2978% UNH 517.55 0.7142% NVO 134.66 1.3091% WMT 59.83 -0.0501% LVMUY 171.22 -0.4072% XOM 118.58 0.7733% LVMHF 856.99 -0.1538% MA 458.0 0.8366%

Annovis Bio Inc
Annovis Bio, Inc. (NYSE: ANVS) is a clinical-stage drug platform company addressing neurodegeneration such as Alzheimer’s disease (AD), Parkinson’s disease (PD) and other chronic neurodegenerative diseases. The Company is developing its lead product candidate, Buntanetap, which is designed to address AD, PD, and potentially other chronic neurodegenerative diseases. Buntanetap is a synthetically produced small molecule, orally administered, brain penetrant compound. Its product candidate ANVS405 is an intravenous drug being developed for acute indications and focused on protecting the brain after traumatic brain injury (TBI) and/or stroke.
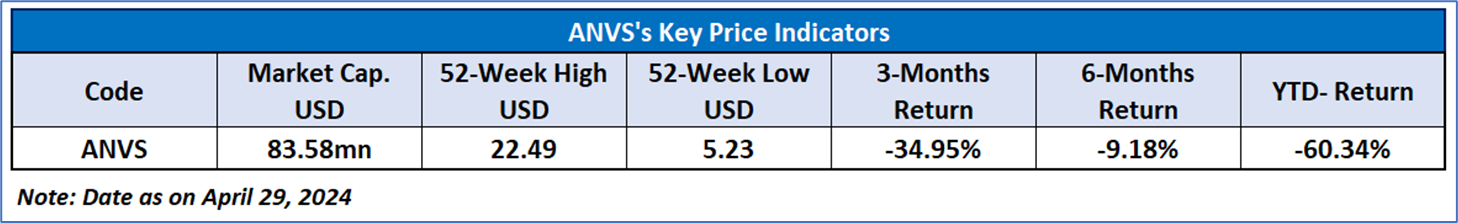
Recent Financial and Business Updates:
Xxxxxx Xxxxxx Xxxxxx
Xxxxxx Xxxxxx
Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx .
Xxxxxx Xxxxxx Xxxxxx Xxxxxx & Xxxxxx
Xxxxxx Xxxxxx
Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx .
Xxxxxx
Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx Xxxxxx .
Enter your details below and our team will help you to decide if this product is best for you.
Kalkine Equities LLC provides general information about companies and their securities. The information contained in the reports, including any recommendations regarding the value of or transactions in any securities, does not take into account any of your investment objectives, financial situation or needs. Kalkine Equities LLC is not registered as an investment adviser in the U.S. with either the federal or state government. Before you make a decision about whether to invest in any securities, you should take into account your own objectives, financial situation and needs and seek independent financial advice. All information in our reports represents our views as at the date of publication and may change without notice.
Kalkine Media LLC, an affiliate of Kalkine Equities LLC, may have received, or be entitled to receive, financial consideration in connection with providing information about certain entity(s) covered on its website.